Food Labeling Errors That Can Cost You Your Business*
*and how to avoid them
Designing a food label can be a daunting task for an entrepreneur bringing a new product to market. Even after you check the boxes for nutrition facts, product claims, and display requirements, you still have to make it work with your packaging design. But if you don’t have time to make sure your label is 100% compliant with every last regulation, then at least avoid the following mistakes — they could cost you your business.
1. Undisclosed Allergens
Many FDA food recalls are related to undeclared allergens. This typically is the result of two easily-avoidable mistakes: (1) the ingredients listing on the package doesn’t match the product in the packaging and (2) The allergens in the product are not displayed prominently to the consumer.
How to Avoid This Mistake: Make sure your ingredients list is complete and that you are identifying any of the Big 8 allergens using an Allergen statement, such as “contains peanuts”.
Who Cares? The FDA, the person you made sick.
Likelihood of Discovery: High. Food allergies are common and undeclared allergens tend to get discovered when a consumer has an allergic reaction to the product, despite having checked the label first.
What it will cost you: Because of the potentially life threatening nature of an allergic reaction to food, food manufacturers typically conduct an immediate, voluntary recall of all effected products.
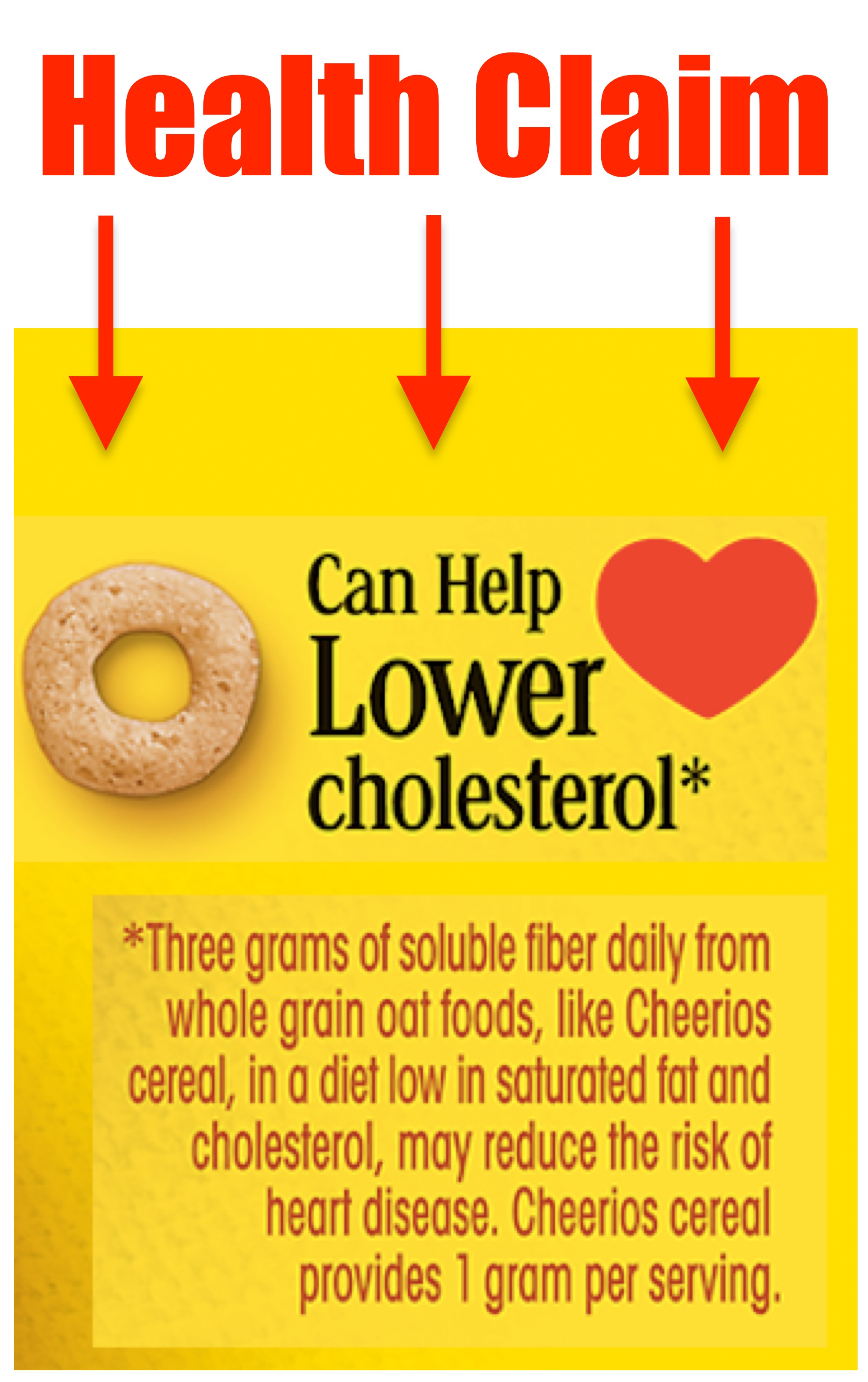
2. Unauthorized Health Claims
There are clearly defined rules when it comes to the types of claims you can make about a food product’s effect on the consumer’s health. The easiest way to guarantee the wrath of the FDA and a swift food recall is to make an-unauthorized health claim or drug claim. This means making a claim about how the product or one of its ingredients affects your body or provides some sort of therapeutic affect. It is unlawful to make this type of claim on the product label itself but also on marketing materials (e.g. the company website), even if you have scientific evidence to back it up.
While the FDA has approved a small number of Authorized health claims approved for use, there are only twelve of them, plus a handful of weakly phrased qualified health claims.
How to Avoid This Mistake: Do not make any unauthorized claim about your product’s effect on the body or treatment of any ailment. Make sure any claims related to nutrition or the benefits of specific ingredients align with the regulation
Who Cares? The FDA. Here’s why: let’s say you claim that the high levels of antioxidants in your popular snack bar has been shown to support immune health in cancer patients. If cancer patients forsake treatment measures in favor of your snack bar, this could have negative public health outcomes. Major retailers will also pull these products from their shelves if they don’t discover this error before they place their order.
Likelihood of Discovery: High. Claims are typically printed prominently to get the consumer’s attention. This also means getting the attention of regulators and savvy consumers.
What it will cost you: All of the products and materials containing the unauthorized claim, possibility of brand damage and the possibility of expensive lawsuits from customers who suffered damages due to this claim..
3. No Standard of Identity (or a misleading one)
FDA labeling requirements clearly require most packaged foods to declare what the product is. This is why a Tostito’s label must clarify that the product is Tortilla Chips and Nutella must describe itself as Hazelnut Spread. While there is a great deal of controversy and open questions surrounding naming conventions for milk-alternatives and meat-alternatives, this isn’t the case for most products.
How to Avoid This Mistake: Include the common name for your product on the front label.
Who Cares? Major retailers who don’t want to be held accountable for carrying a product which could be construed as misleading.
Likelihood of Discovery: Medium-High. It may not be discovered initially, but eventually someone will notice. Typically, a failure to disclose a product’s Standard of Identity doesn’t have negative health implications for the consumer, it’s just mildly confusing, an infraction which rates lower on the FDA’s list of priorities. It’s more likely that a wholesale purchaser or retailer will find issue with this labeling error than the FDA.
What it will cost you: Mainstream retailers may refuse to carry the product until this issue is remedied.
4. Undeclared Ingredients
Even when there’s no allergen implications, the discovery of an undeclared ingredient in a product can bring public outrage to a boil and even lead to a food scare. Quite simply, consumers don’t like finding out that they have been lied to, particularly about what’s in their food.
How to Avoid This Mistake: Work with trusty suppliers and conduct supplier verification activities so you can have confidence in the ingredients you purchase. Make sure that your food labels and ingredients reflect changes made to the product formula.
Who Cares? Your most loyal customers; Twitter.
Likelihood of Discovery: Low-Medium. Using the final few rolls of old food labels after a slight change in product formulation will likely go unnoticed. The deliberate, ongoing failure to accurately convey ingredient composition is more common than we know. Large-scale food fraud can go undetected for years before being uncovered and technology is sometimes altogether incapable of detecting when ingredients are replaced with a cheaper alternative.
What it will cost you: The ongoing failure to accurately declare ingredient composition could result in lawsuits, fines, and total destruction of your brand.